Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Crystallization and Flowability Process Scale-Up, a Quality by Design (QbD) Perspective: Sodium Citrate
Authors: L. Srinivasula Reddy, Prof. Dr. A. G. Damu, V. Manohar Reddy
DOI Link: https://doi.org/10.22214/ijraset.2024.63972
Certificate: View Certificate
Abstract
Crystallization process scale?up is extremely challenging and this credit summarizes a QbD (Quality by Design) approach for robust crystallization process development in addition to Flowability. To avoid common issues inconsistency of flowability, crystallinity of material, QbD approach has been developed for the manufacturing of Sodium citrate, which is used as excipient (pharmacologically inactive substance). To improve crystallization and flowability of material, the effect of reaction mass (RM) Temperature, effect of addition rate and time, effect of different RPM, effective of equipment selection has been studied and results discussed in this research paper.
Introduction
I. INTRODUCTION
Sodium citrate is the sodium salt of citric acid. It has a sour taste similar to citric acid and is salty as well. It is often used as a food preservative, and it is a flavoring in the food industry. In the pharmaceutical industry, this salt is used as an excipient and to control pH during drug manufacturing process., also it may be used as an alkalizing agent, buffering agent, emulsifier, or sequestering agent. [1]. According to the GRAS (USFDA Select Committee on Generally Recognized as Safe) food substances, citrate salts, including sodium citrate, are generally regarded as safe when used in normal quantities. [2]. Sodium citrate is the sodium salt of citric acid used to neutralize excessive acidity (metabolic acidosis) in the body, and prevent the formation of kidney stones (nephrolithiasis). Sodium citrate increases systemic and urinary pH levels and also increases the excretion of urinary calcium, inhibiting the formation of calcium stones in the kidneys. This drug is combined with citric acid and marketed labeled as Sodium Citrate and Citric Acid Oral Solution USP [3]. citrate therapy in chronic renal failure may have positive effects on salt sensitivity and ameliorate the progression of kidney injury, and that these effects could be related to the altered expression of NHE3 and correction of metabolic acidosis. These results suggest that alkali therapy might be applied to other salt-sensitive pathologic conditions as well as chronic renal failure [4]. The chemical formula of sodium citrate is Na3C6H5O7. The chemical structure of Sodium citrate is shown in Figure 1.
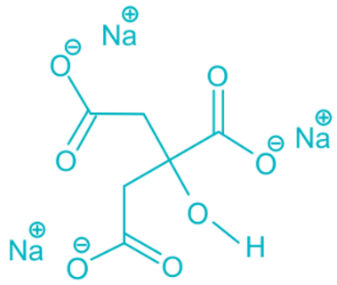
Figure 1: Chemical structure of Sodium citrate
A. Preparation of Sodium Carbonate
In general, sodium citrate is ordinarily made by adding sodium carbonate monohydrate to a hot aqueous solution of citric acid. The resulting solution is then evaporated until crystallization takes place. A further method for producing sodium citrate is by decomposing calcium citrate by means of an alkali metal salt. A Chinese patent [5] disclosed, preparation method of sodium citrate crystal through solvent-out crystallization procedure, in this invention, carrying out salt-forming reactions: and carrying out solvent-out crystallization, filtering and drying. So, as to obtain the sodium citrate product; wherein the sodium citrate product comprises waterless sodium citrate, dihydrate sodium citrate, and pentahydrate sodium citrate. The generalized process scheme of Sodium citrate preparation is shown in Figure 2.
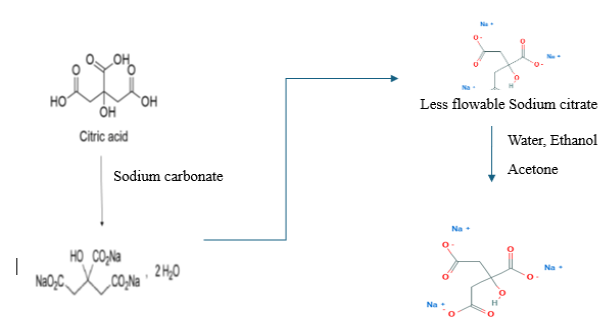
Figure 2: Process preparation of Sodium citrate
Quality by design (QbD) is a concept first developed by the quality pioneer Dr. Joseph M. Juran [6].The concepts behind quality by design were introduced in international pharmaceutical guidelines intended for the pharmaceutical industry between year 2009 and 2012.The main key goal of quality by design is to ensure that all sources of variability affecting a process are identified, explained and managed by appropriate measures. In the context of pharmaceuticals, Quality by Design focuses on optimizing the development, manufacturing, and control processes of drugs to enhance their safety, efficacy, and overall quality. It requires a deep understanding of the product's critical quality attributes (CQAs), which are the measurable characteristics that determine its performance, and the critical process parameters (CPPs), which are the variables affecting the manufacturing process. Over the years, pharmaceutical QbD has evolved with the issuance of ICH Q8 (R2) (Pharmaceutical Development), ICH Q9 (Quality Risk Management), and ICH Q10 (Pharmaceutical Quality System) [7-9].
II. OBJECTIVE OF CRYSTALLIZATION PROCESS:
The objective of this research publication is discussed how to improve the excipient flowability and crystallinity during the sodium citrate crystallization process of excipient by conducting the experiments and verification of a design space at Reaction mass (RM) temperature, equipment selection, addition rate and time, equipment different RPM. To produce the desired results of flowability and crystallinity. Finally, to improve the process efficiency and overall process robustness without compromising quality standards.
Crystallization is a process of formation of solid crystal from a uniform solution. Its widespread use salt, since in a single operation enables both separation as well as purification, resulting in a crystalline solid which is sequestered with high level of purity and low capital and operating cost. Crystallization process provides the chance to govern the size and number of crystals formed. In the case of pharmaceutical industries, the size and structure of the crystals formed are significant.
Product quality and efficiency are often dependent on satisfactory control of particle size and structure. The size of crystal produced in a crystallizer can have an unfavorable effect on downstream operations. Therefore, it is desirable to propose a crystallization process that prevents extreme fines generation and thus can reduce downstream operation problems. The degree of super saturation can be calculated using the population balance together with the understanding of the nucleation and growth kinetics. The population balance method has a vital part in crystallization and other particulate processes.
Crystallization is the solidification of atoms or molecules into a highly structured form called a crystal. Crystallization can also refer to the solid-liquid separation and purification technique in which mass transfer occurs from the liquid solution to a pure solid crystalline phase. The following flow chart represents the crystallinity procedure.
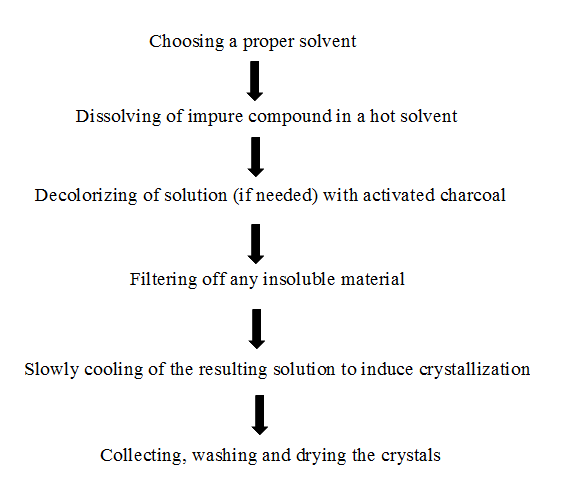
Review of crystallization and flowability experiments, it is being refers to the technology transfer and execution of a process at a scale by approximately one order of magnitude larger than the previous experimentation scale, with focus on the current execution of the process in a pilot or in a manufacturing plant. There are several reasons and difficulties of crystallization and flowability improvement and observed following significant affecting on the crystallinity and flowability.
Type of Solvent, Equipment selection, Crystallization temperature and cooling, RPM, Surface of the crystal vessel and Addition time and rate. According to these parameters, process optimization can be achieved.
A. Improvement of Crystallization and increase the flowability by using the QbD technic.
As discussed in previous sections, QbD approach advances us to make a clear distinction in the process development between scale-independent and scale-dependent process parameters. Because the processes development required multiple analysis, such as statistical design of experiments (DoE), is a more appropriate experimental methodology than one-variable-at-a-time investigations. It is preferred to first define a small-scale design space using only scale-independent parameters. In order to accomplish this, we start with a team brainstorm to generate ideas an exhaustive list of all the process parameters impacting the product CQAs, crystallinity and flowability. Such lists tend to be relatively long, perhaps six parameters. The next step will be to conducted experiments by using the statistical DOE.
To keep the screening DoE matrix at a practical size, first need to prioritize the brainstormed list and select a suitable number of parameters to be included in the screening DoE study. This ranking step also contributes to the preliminary risk analysis. Several methods can be used to rank the process parameters, with Kepner-TregoeTM and FMEA (failure mode and effects analysis) being among the most popular. Although FMEA tends to be more familiar to process scientists from hazards analysis, Kepner-TregoeTM [10] tends to be faster.
The ensuing DoE experimentation will confirm the validity of the ranking or, occasionally, it will show that a certain process parameter was wrongly excluded from the screening study. Frequently used types of DoE-screening matrixes are fractional and full factorial designs, with occasional use of more aggressive designs such as Plackett-Burman [11].
The statistically significant parameters identified in the DoE screening study can then be included in an optimization or RSM (response surface methodology) study in order to develop a small-scale design space. A separate set of QbD experiments, described in more detail below, aims at the understanding of the parameter change impact on process results. During such scale-up investigations, we attempt to understand the controlling mechanism for mixing and identify a scale-up factor. With the rare exception of mixing insensitive crystallizations, flowability to complete process model includes both scale-independent and scale-dependent factors.
III. EXPERIMENTAL RESULTS AND DISCUSSION
After brainstorming and root cause analysis, risk assessment, literature process review understood that the following probable factors there may impact on flowability and crystal formation during addition and pH adjustment.
- Addition temperature.
- Addition time and rate.
- Equipment selection.
- Equipment different RPM.
- Addition solvent selection, quantity.
Based on the above identified probable affecting factors the below experiments have been conducted and discussed experiment wise:
A. Experiment – 1: (as such experiment)
The existing procedure was taken into account for evaluation. About 100 grams of sodium citrate impure material was taken as a input and it is dissolved in water at room temperature. After dissolution, treatment with carbon followed by filtration. After filtration addition of solvent mixture i.e., Ethanol and acetone mixture to sodium citrate at 25-35ºC, adjust pH with diluent NaOH and maintain for 1 hour at 33-35ºC. Filtration can be performed followed by drying at 1hour 5 min without agitation at 40-50ºC. After completion 1 hour 5 min drying, raise the temperature to 56-60ºC and continue drying up to 19 to 20 hours. Ethanol and Acetone were quantified in GC method and bulk density was done with tap density. The obtained values are shown in Table 1.
|
Experiment No |
Batch No |
Input (g) |
Temperature (°C) |
Agitator |
RPM |
Yield (%) |
GC (ppm) |
BD |
|
|
Ethanol |
Acetone |
||||||||
|
1A |
SOM/EXP/1 |
100 |
25-35 |
PBT |
360 |
0.93 |
46 |
95 |
0.47 |
Table 1: As such method results from Experiment-1
The above experiment indicates that reaction is exothermic, when addition of ethanol and acetone mixture into Sodium citrate solution during nucleation (~2-3°C) and agglomerated crystals have been observed after complete addition of Ethanol-Acetone mixture based on microscopic images data. Microscopic images are shown in Figure 3. Based on this experimental data, agglomerated crystals might cause scaling or lump formation in scale up batches and will impact on material flowability. Hence, further experiments have been conducted on effect of ethanol & acetone mixture addition temperature, addition rate and Effect of ethanol & acetone mixture addition time, Effect of RPM by considering the change of addition temperature and addition time, addition rate, RPM and remaining equipment selection, additional solvent selection to avoid the scaling or lump formation during crystallization and increase the flowability.
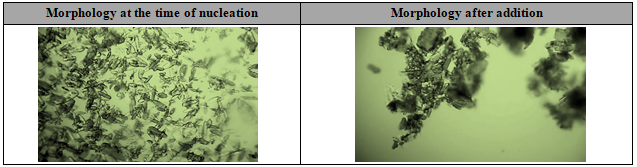
Figure 3: The morphology observation for Experiment-1
B. Experiment -2 (Effect of Ethanol & Acetone mixture addition temperature)
During the Ethanol and acetone solvent addition, temperature has been changed (within the range i.e existing process temperature i.e., 14-35°C) for each experiment., however no change in addition time and RPM. A total of four experiments were conducted. In all experiments, after 30% addition, nucleation started with agglomerated crystals at various temperatures. Each experimental outcome has conducted microscopic and observed all four experimental photos. By comparing all images more agglomerated were observed in 2A and 2B experiments and less agglomerated were observed in experiment 2C and 2D. Experimental details are shown in Table 2 and microscopic images are shown in Figure 4.
|
Experiment No. |
Batch No |
Input (g) |
Addition Temperature (°C) |
GC (ppm) |
Yield (%) |
BD
|
Remarks
|
||
|
Ethanol |
Acetone |
||||||||
|
2A |
SOM/EXP/02 |
100 |
14-20 |
126 |
54 |
0.96 |
0.43 |
After 30% addition, nucleation started with agglomerated crystals and temperature raised from 14°C to 20.6°C. |
|
|
2B |
SOM/ EXP /03 |
100 |
20-25 |
59 |
12 |
0.84 |
0.60 |
After 30% addition, nucleation started with agglomerated crystals and temperature raised from 21.2°C to 23.6°C. |
|
|
2C |
SOM/ EXP /04 |
100 |
25-30 |
40 |
95 |
0.93 |
0.47 |
After 30% addition, nucleation started with agglomerated crystals and temperature raised from 28.1°C to 29.2°C. |
|
|
2D |
SOM/ EXP /05 |
100 |
30-35 |
100 |
36 |
0.88 |
0.54 |
After 30% addition, nucleation started with agglomerate crystals and temperature raised from 31.4°C to 34.2°C. |
|
Selected RPM: 360 RPM and Pitch Blade Turbine Agitator in 1L reactor. Ethanol-Acetone solution was added in 100-120 min in all the above four experiments.
Table 2: Results and observations from Experiment-2
|
Experiment Number |
Microscopic Images |
Remarks |
|
2A |
|
After 30% addition, nucleation started with agglomerated crystals and temperature raised from 14°C to 20.6°C. |
|
2B |
|
After 30% addition, nucleation started with agglomerated crystals and temperature raised from 21.2°C to 23.6°C. |
|
2C |
|
After 30% addition, nucleation started with agglomerated crystals and temperature raised from 28.1°C to 29.2°C. |
|
2D |
|
After 30% addition, nucleation started with agglomerate crystals and temperature raised from 31.4°C to 34.2°C. |
Figure 4: The morphology observation for Experiment-2
C. Experiment -3 (Effect of Ethanol-Acetone mixture addition time)
In this experimental series, a total five number of experiments were conducted by varying the addition time from 15 min to 390 min range and observed the addition time effect on crystallization. All these five experiments, addition temperature, RPM were same. In experiment (3A), experiment (3B) and experiment (3C) were observed scale formation on agitator along with agglomerated crystals. Moreover, all five experiment output samples were conducted microscopic images and observed more agglomerated was observed. Whereas in the remaining two experiments (3D & 3E) were not observed no agglomerated and scale formation. For more experimental details are captured below Table 3 and microscopic images are shown in Figure 5.
|
Experiment |
Batch No |
Input (g) |
Time (min) |
Yield (%) |
GC (ppm) |
BD g/ml |
Remarks |
|
|
Ethanol |
Acetone |
|||||||
|
3A |
SOM/ EXP/06 |
50 |
15 |
0.91 |
80 |
28 |
0.47 |
Scaling observed for agitator along with agglomerated crystals during Ethanol-acetone addition. |
|
3B |
SOM/EXP /07 |
50 |
120 |
0.93 |
46 |
100 |
0.47 |
Mild scaling observed for agitator along with agglomerated crystals during Ethanol-acetone addition. |
|
3C |
SOM/EXP/08 |
50 |
240 |
0.90 |
61 |
44 |
0.53 |
Mild scaling observed for agitator along with less agglomerated crystals during Ethanol-acetone addition. |
|
3D |
SOM/EXP/09 |
50 |
390 |
0.87 |
10 |
12 |
0.5 |
No scaling and no agglomerated particles observed. |
|
3E* |
SOM/EXP/10 |
50 |
315 |
0.94 |
ND |
8 |
0.7 |
No scaling and no agglomerated particles observed. |
Selected RPM: 360 with Pitch Blade Turbine (PBT) in 1L reactor, addition temperature of ethanol and acetone mixture at 25ºC to 35ºC.
*Experiment No.3E conducted in 5 L Reactor with 270 RPM with PBT.
Table 3: Results and observations from Experiment-3
|
Experiment Number |
Microscopic Images |
Remarks |
|
3A |
|
Agglomerated crystals observed during Ethanol-acetone addition. |
|
3B |
|
Agglomerated crystals observed during Ethanol-acetone addition. |
|
3C |
|
Agglomerated crystals observed during Ethanol-acetone addition. |
|
3D |
|
No agglomerated crystal particles observed. |
|
3E* |
|
No agglomerated crystal particles observed. |
Figure 5: The morphology observation for Experiment-3
D. Experiment -4 (Experiments conducted with different RPM)
Based on the mixing calculations, conducted three experiments by varying the RPM (from 270-360RPM). All these experiments were conducted at addition temperature 25ºC to 35ºC and addition time 5-6 hours. Experimental data is shown in Table 4.
|
Experiment |
Batch No |
Input (g) |
Addition time (h) |
RPM |
Yield (%) |
GC (ppm) |
BD (g/ml) |
Remarks |
|
|
Ethanol |
Acetone |
||||||||
|
4A |
SOM/EXP/11 |
250 |
5.15 |
270 |
0.94 |
ND |
8 |
0.7 |
No scaling and no agglomerated particles observed. |
|
4B |
SOM/EXP/12 |
250 |
5.5 |
200 |
0.94 |
25 |
6 |
0.74 |
No scaling and no agglomerated particles observed. |
|
4C |
SOM/EXP/13 |
250 |
5.5 |
360 |
0.95 |
8 |
2 |
0.71 |
No scaling and no agglomerated particles observed. |
Experiments conducted Pitch Blade Turbine Agitator in 5L reactor. with addition temperature at 25-35°C.
Table 4: Results and observations from Experiment-4
|
Experiment Number |
Microscopic Images |
Remarks |
|
4A |
|
No agglomerated crystal particles observed. |
|
4B |
|
No agglomerated crystal particles observed |
|
4C |
|
No agglomerated crystal particles observed. |
Figure 6: The morphology observation for Experiment-4
From the above experiments, it has been observed that solvent mixture addition at lower temperature i.e. 14-25°C is leading to form more agglomerated crystals during nucleation compared to addition at 25-35°C. Hence, it is recommended to add the Ethanol- Acetone mixture solution at 25-35°C. from the experiment-3 fast addition of Ethanol& acetone at 25-35°C leading to form agglomerated crystals during nucleation when compared to slow addition. Hence, it is recommended to add the Ethanol- Acetone solution to reaction mass for 5-6 h at 25-35°C and there is no impact on crystallization based on experiment 4 results.
E. Experiment -5 (Finalized Methodology)
From the above QbD approach experiments, by considering all the parameters, the following methodology has been finalized and implemented for scale-up.
250 grams of sodium citrate was taken as input material, and it is dissolved in water at room (i.e. 20-30ºC). After dissolution of the material, it is treated with carbon and followed by filtration. After filtration, addition of solvent mixture (i.e. Ethanol and acetone mixture) to sodium citrate at 25-35ºC in the period of 5-6 hours, adjust pH with diluted Sodium hydroxide and maintain for 01 hour at 33-35ºC. After filtration, it is drying at 45-50ºC for 1 to 1hour 5 minutes without any agitation at. After drying raise the temperature to 56-60ºC and continue drying 19 to 20 hours.
Finalized process experimental data:
|
Experiment |
Batch No |
Input (g) |
Time (min) |
Yield (w/w) |
GC (ppm) |
Remarks |
|
|
Ethanol |
Acetone |
||||||
|
5A |
SOM/EXP/14 |
250 |
315 (5.15 h) |
0.94 |
ND |
10 |
No scaling and no agglomerated particles observed. |
|
5B |
SOM/EXP/15 |
250 |
330 (5.30 h) |
0.95 |
ND |
7 |
No scaling and no agglomerated particles observed. |
Table 5: Results and observations from Final methodology Experiment-5
|
Experiment Number |
Microscopic Images |
Remarks |
|
5A |
|
No agglomerated particles observed. |
|
5B |
|
No agglomerated particles observed |
Figure 7: The morphology observation in finalized methodology for Experiment-5
Conclusion
Based on the QbD approach experiments data and morphology observations, in the process preparation of Sodium citrate during crystallization, it has been confirmed that, addition time, rate and temperature were major affecting factors on flowability and crystal formation for scale-up activity.
References
[1] Dave RH. Overview of pharmaceutical excipients used in tablets and capsules. Drug Topics (online). Advanstar. 2008. http://drugtopics.modernmedicine.com/drugtopics/Top+News/Overview-of-pharmaceutical-excipients-used-in-tabl/ArticleStandard/Article/detail/561047. [2] FDA’s SCOGS database; triethyl citrate; SCOGS-Report Number: 84; 2012, http://www.accessdata.fda.gov/scripts/fcn/fcnDetailNavigation.cfm?rpt=scogsListing&id=356 [3] Sodium Citrate and citric acid- sodium citrate and citric acid monohydrate solution Cardinal Health 107, LLC [4] Sejoong Kim, Jin Young Yang, Eun Sook Jung, Jeonghwan Lee, Nam Ju Heo, Jae Wook Lee, Ki Young Na, and Jin Suk Han,Effects of Sodium Citrate on Salt Sensitivity and Kidney Injury in Chronic Renal Failure, J Korean Med Sci. 2014 Dec; 29(12): 1658–1664. [5] CN104262139A, China Patent, Preparation method of sodium citrate crystal through solvent-out crystallization, North China Pharmaceutical Group Xiantai pharmaceutical Co Ltd, 2014 [6] Juran JM. Juran on quality by design: the new steps for planning quality into goods and services. New York: The Free Press; 1992. [Google Scholar] [7] U. S. Food and Drug Administration. Guidance for Industry: ICHQ8 (2) Pharmaceutical Development. 2009 [8] U. S. Food and Drug Administration. Guidance for Industry: ICHQ9 Quality Risk Management. 2006. [9] U. S. Food and Drug Administration. Guidance for Industry: ICHQ10 pharmaceutical quality system. 2009. [10] Myerson, A. S. Handbook of Industrial Crystallization; Butterworth- Heinemann: Oxford, 2001. [11] Tung, H.-H.; Paul, E. L.; Midler, M.; McCauley, J. A. Crystallization of Organic Compounds: An Industrial Perspective; John. Wiley & Sons, Inc.: New York, 2009.
Copyright
Copyright © 2024 L. Srinivasula Reddy, Prof. Dr. A. G. Damu, V. Manohar Reddy. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET63972
Publish Date : 2024-08-14
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online















